Despite what some climate change deniers might have you think, the build-up of carbon dioxide in the atmosphere is measurably and quantifiably the result of human emissions. We know the concentration of carbon dioxide in the atmosphere is increasing. Figuring out where it’s coming from requires understanding the carbon cycle and the different types of carbon atom on Earth.
Human emissions of CO2 are part of the carbon cycle, a global geochemical interconnected web of carbon sources (reactions that add carbon to the atmosphere) and sinks (reactions that sequester carbon in the sea, land, or biomass). For thousands of years before the industrial revolution, all the natural sources of CO2 were balanced out by an equal amount of sinks. Without an increase in sinks, any extra amount of CO2 added by humans was inevitably going to hang around in the atmosphere (though to be clear, a not insignificant amount of human emissions does get reabsorbed, just not all of it).
Additionally, not all carbon atoms are the same, although it’s pretty close. To begin with, 100% of all carbon atoms in the universe have 6 protons in their nucleus. It is these 6 protons that define carbon. Every chemical element is defined by the number of protons in its atoms’ nuclei, as the number of positively-charged protons determines how strongly the nucleus attracts the much smaller, negatively-charged electrons.
Atomic nuclei can also contain the chargeless neutrons (in fact for increasingly large numbers of protons, neutrons are necessary to keep the nucleus stable), and the isotopes of a chemical element are the different ways to build an atom of that element with different numbers of neutrons. For example, around 98.9% of all carbon atoms on Earth have 6 protons and 6 neutrons. We call this isotope carbon-12 (denoted 12C). We often hear about carbon-14 (denoted 14C) in archaeology. This isotope has 8 neutrons, and is radioactive and unstable, decaying over time and quite rapidly (at least, in geological terms it’s very rapid).
But 14C and other unstable isotopes of carbon exist in less than one part per trillion on Earth. Carbon-13 (13C) on the other hand, is both stable (i.e. it does not decay over time) and accounts for approximately 1.1% of Earth’s carbon. And while the difference is very very slight, the fact that carbon-13 is heavier than carbon-12 leads to small but measurable differences in their chemistry.
While chemical reactions will occur almost the same with 13C as 12C, it’s not exact. A striking example of this in climate science is that 12C, being lighter, is more readily taken up from the air by land plants during photosynthesis. Plants remove more 12C from the atmosphere than they remove a proportionate amount of 13C (that is, less than 1.1% of carbon absorbed by plants is 13C), and they build that carbon into their biomass.
In scientific literature, the symbol δ13C represents the isotopic signature of 13C, a value that reflects the measured abundance of 13C relative to the amount of 12C in some sample of carbon, be it a pocket of air or a lump of rock. We can thus say that the data show that δ13C for the atmosphere is higher than the δ13C for plants.
Which means that as we burn fossil fuels derived from terrestrial plants, such as coal, then the amount of 13C emitted relative to 12C is less than that which we would find if the δ13C for fossil fuels was the same as the δ13C for the atmosphere. We’re effectively tilting the scales toward carbon-12, i.e. increasing the denominator in the ratio, i.e. decreasing δ13C.
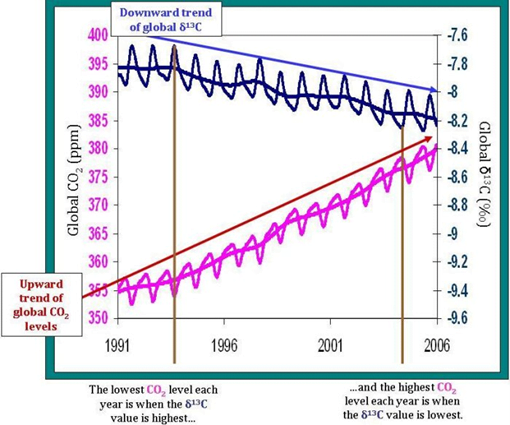
By directly observing and measuring the δ13C content of fossil fuels and the amount of fossil fuels being burned, we have a quantifiable measure of how much 13C is being added to the atmosphere relative to 12C. When compared with the measurements of declining δ13C, we can see these two measurements line up.
Of course, not all fossil fuels have the same carbon isotope signatures. Coal derived from terrestrial plants is more depleted in 13C than oil derived from marine plankton. But the principal of different isotopes leaving unique signatures in different reactions applies everywhere. A recent paper even used an isotope of helium, 4He, to track the impact of natural gas on the atmosphere.
When these isotope signatures are combined with the measurements made of carbon emissions, the conclusions are inescapable. Any hypothetical increase from a natural source of CO2 could also cause a buildup in the atmosphere, but, on top of there being no significant increase in natural carbon emissions, none of the existing sources could explain the δ13C decrease. Fossil fuels are the only culprit that fit the data.



